A net ionic equation includes the ions that are involved in the formation of the solid.
In this case, the solid is silver chloride (AgCl). The ions that are involved are Ag+ and Cl-, then, the net ionic equation is:
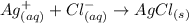
It means that the correct answer is the last choice.