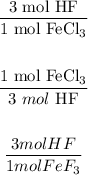Answer:
We have the following chemical equation
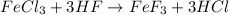
We can see that the equation is balanced because we have the same number atoms of each element on each side of the equation.
So now we can answer, the mol ratios that correspond to the balanced equation are: