To determine if the molecule is polar or non polar, we have to find the difference of the electronegativities of the elements.
Ec has an electronegativity of 3.4 and F an electronegativity of 3.98:
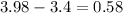
If the difference is between 0.4 and 1.7 the molecule is polar, when it is less than 0.4 it is non polar.
According to this, the given molecule is polar.
The given molecule also is symmetrical (it has a symmetry axis) and has polar bonds.