Molarity is a way of expressing the concentration of a solute dissolved in a solvent. It involves the moles of the solution and the total volume of the solution. Molarity is expressed as moles of solution per liter of solution, so the molarity equation will be:
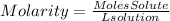
Therefore, the answer will be d. Moles of solute (mol) ÷ liters of solution (L)