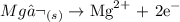Answer:
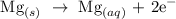
Step-by-step explanation:
Here, we want to write a balanced half-cell reaction for oxidation
Firstly, we have to identify the species being reduced (they are the ones that undergo oxidation)
Oxidation usually involves the loss of electrons. From what we can see, Magnesium lost 2 electrons to become positively charged
Thus, we have the oxidation half-reaction as: