Answer
Volume of water = 7.35 L
Step-by-step explanation
Given:
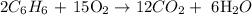
Volume of benzene = 2.45 L
Pressure = 1.25 atm
Temperature = 25 C = 298 K
Required: Volume of water vapour that will be formed
Solution
Step 1: Calculate the number of moles of benzene using ideal gas law
PV = nRT
n = PV/RT
n = (1.25 atm x 2.45 L)/(0.08206 L.atm/K.mol x 298 K)
n = 0.125 mol
Step 2: Use the stoichiometry to find the moles of water
The molar ratio between benzene and water is 2:6
Therefore moles of water = 0.125 mol x (6/2) =0.376 mol
Step 3: Calculate the volume of water that will be formed
V = nRT/P
V = (0.376 mol x 0.08206 L.atm/K.mol x 298 K)/1.25 atm
V = 7.35 L