Step-by-step explanation
Given
N2(g)+3H2(g)—> 2NH3(g)
Number of moles of nitrogen = 1.5 moles
Number of moles of hydrogen = 6 moles
Required: Limiting reagent
Solution
For Nitrogen:
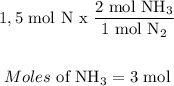
For Hydrogen:
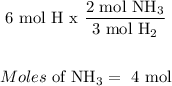
The limiting reagent is Nitrogen, because less moles of NH3 will be produced by nitrogen, meaning it all of it gets used up.
Answer
The limiting reagent is Nitrogen.