In order to calculate the final temperature, we can equate the heat equation of one water to minus the heat of the other water, this way the hotter water will give energy to the colder water. So we have:
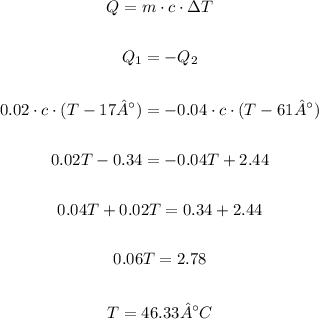
Therefore the final equilibrium temperature is 46.33 °C.