Boyle's Law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its pressure.
Most gases behave like ideal gases at moderate pressures and temperatures.
We use this:
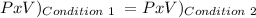
T doesn't change.
Condition 1:
P1 = 17 psi
V1 = 6.563 L
Condition 2:
P2= We need to find this
V2 = 1 L
-----------------------------------------------------------------------------------------
From Boyle's Law we clear P2:
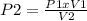
P2 = (17 psi x 6.563 L) / 1 L = 111 psi
Answer: P2 = 111 psi (condition 2)