ANSWER
The reaction will occur spontaneously
YES
Step-by-step explanation
Given information
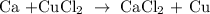
The above reaction is an example of a single replacement reaction.
The Ca displaces Cu on the left-hand side of the reaction to produce CaCl2.
According to the metal activity series chart, Calcium is more reactive than copper and it will displace copper during a chemical reaction.
Therefore, the reaction will occur spontaneously.