Answer:
•How do you find the delta H per grams in an chemical formula
➢ Subtract the sum of the heats of formation of the reactants from that of the products to determine delta H: delta H = –110.53 kJ/mol – (–285.83 kJ/mol) = 175.3 kJ.
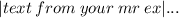
#CarryOnLearning