Answer
Titanium has the above properties because it belongs to group d- block element
Titanium is a transitional metal and has properties of transition metal.
Titanium d-orbital is partially filled having the following electronic configuration
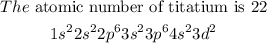
The valency electron is 2 and this shows that it belongs to group 2 and the d - orbital is partially filled.
General properties of d -block elements ( Transition metal)
1. Form stable complexes.
2. Have high melting and boiling points.
3. Contain a large charge/radius ratio.
4. Form compounds that are often paramagnetic.
5. Are hard and possess high densities.
6. Form compounds with profound catalytic activity.
7. show variable oxidation states.
Hence, Titanium has the above properties because it belongs to group d- block element