Answer: 30.6g of KClO3 corresponds to 0.249 moles of this compound.
Step-by-step explanation:
The question requires us to calculate the number of moles that correspond to 30.6 g of potassium chlorate (KClO3).
To determine the number of moles of KClO3, first we need to calculate the molar mass of this compound.
Considering the atomic masses given by the question (K = 39.1 g/mo, Cl = 35.4 g/mol and O = 16.0 g/mol), we can calculate the molar mass of KClO3 as:
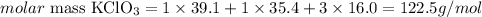
Therefore, the molar mass of KClO3 is 122.5 g/mol.
Next, we need to convert the mass given, 30.6g, to the number of moles, using the molar mass of KClO3:
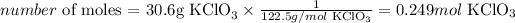
Therefore, 30.6g of KClO3 corresponds to 0.249 moles of this compound.