The question requires us to calculate the theoretical yield of a reaction, given the percent yield (43.3%) and actual yield (7.1g).
The percent yield of a chemical reaction can be calculated as:
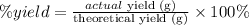
We can rearrange this equation in order to calculate the theoretical yield value. Our first step will be conver the
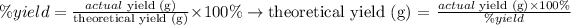
Now, we can apply the values given by the question to the equation above:
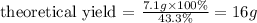
Therefore, the theoretical yield of this reaction would be 16g. Note that the value for actual yield, 7.1g, was given with two significant figures - thus our answer must have the same amount of significant figures.