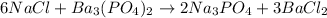
Firstly we must convert mass to moles:
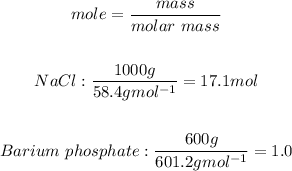
Note: The ratio of given mole compared to the theoretical mole can be used to tell the limiting reactant. The lowest mole per coefficient ratio is the the limiting reactant. The highest is the excess reactant.
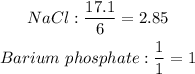
The limiting reactant is barium phosphate