HCl is a strong acid, so in solution, it will release all the H+ ions that are in the molecule. Therefore, the concentration of HCl acid will be equal to the concentration of H+ ions.
The concentration of H+ ions can be found by means of the pH equation that says:
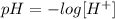
where [H+] is the concentration (Molarity) of H+ ions (Hydronium).
By clearing the concentration of H+ we have:
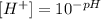
We replace the value of pH into the equation:
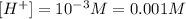
Answer: the hydronium concentration of an HCl solution with a pH of 3.0 is 0.001M