Answer:
1.6moles
Explanations:
The formula for calculating the molarity of a solution is expressed according to the equation:
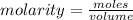
Given the following parameters
volume of solution = 400mL = 0.4L
molarity of the solution = 4M
Determine the moles present
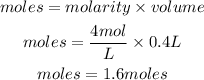
Hence the number of moles present is 1.6moles