Answer
Step-by-step explanation
Note that a mole of any substance has 6.02 x 10²³ atoms of that substance.
Let x represent the mole of magnesium present in 5.01 x 10²² atoms of magnesium.
Therefore,
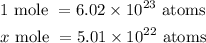
Cross multiply, and divide both sides by 6.02 x 10²³ atoms to get x
![undefined]()