They give us the balanced equation of the reaction.
3 KOH + H3PO4 → K3PO4 + 3H2O
So we can continue with the calculations.
We first find the moles of K3PO4 that will be formed. For that we look at the stoichiometric coefficients of the reaction. The K3PO4 to KOH ratio is 1/3. So the moles of K3PO4 will be:
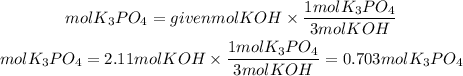
To find the mass we must multiply the moles found by the molar mass of potassium phosphate. The molar mass of potassium phosphate is:212.3g/mol
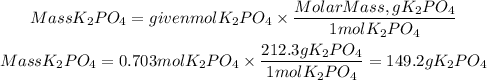
Answer: The mass of potassium phosphate produced is 149 grams