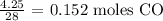Answer:
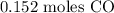
Step-by-step explanation:
Here, we want to get the number of moles in 4.25 g of CO
To get the number of moles, we have to divide the mass by the molar mass of CO
Mathematically:
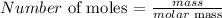
The molar mass of CO is the sum of the atomic masses of carbon and oxygen
The atomic mass of carbon is 12 amu
The atomic mass of oxygen is 16 amu
The molar mass is thus:
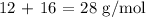
Thus, we have the number of moles as: