ANSWER
2, 1, 2
option D
EXPLANATION
Given that;
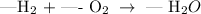
Apply the law of conservation of mass to balance the given chemical equation
Law of conservation of mass states that matter can neither be destroyed nor created but can be transformed from one form to another
Assign letters to each of the reactant and product
We have,
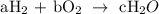
2a = 2c
2b = c
since c = 2b
Hence,
2a = 2(2b)
2a = 4b
Recall, 2a = 2c
2c = 4b
c = 4b/2
c = 2b
Hence, a = 2, b = 1 and c = 2
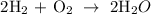
Therefore, the correct answer is 2, 1, 2 ------ option D
_