To determine what type of bond is formed when hydrogen and oxygen form water, we have to find the difference in electronegativities of hydrogen and oxygen:
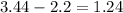
According to this, the difference between electronegativities is less than 1.7 and greater than 0.4, which means that the type of bond formed by oxygen and hydrogen is polar covalent bond.