Answer:
1.13 atm
Step-by-step explanation:
The new pressure can be found by using the formula
P1V1 = P2V2
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
Since we're finding the new pressure P2 we make P2 the subject
We have
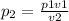
P1 = 1.7 atm
V1 = 0.6 L
V2 = 0.9 L
We have
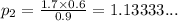
We have the final answer as
1.13 atm
Hope this helps you