Answer:
1: London Dispersion Forces. Iodine molecules are non polar, so they do not have dipole-dipole attractions or hydrogen bonding.
2. Hydrogen bonding. Water contains hydrogen atoms bonded to the highly electronegative oxygen atom, thus resulting in the hydrogen atoms being highly polarized. This causes hydrogen bonding.
3. Electrostatic attraction (ion-ion). Because NaI is an ionic compound, the
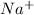 ions within its lattice are attracted to the negatively charged
ions within its lattice are attracted to the negatively charged
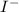 ions.
ions.
4. London Dispersion Forces. Because carbon and hydrogen do not differ greatly in electronegativity,
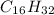 is considered to be non polar and thus does not have hydrogen bonding or dipole-dipole attractions.
is considered to be non polar and thus does not have hydrogen bonding or dipole-dipole attractions.