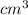Answer:
19.29 g/
Step-by-step explanation:
Gold nugget - what we know:
Density's formula: (g/
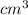 )
)
Gold volume: 1.68
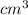
Gold mass: 32.4 g
Use the density formula to find the density of the gold nugget:
= (g ÷
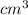 )
)
= (32.4 ÷ 1.68)
= 19.285 g/
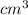
Therefore the gold nugget has a density of 19.29 g/