Answer:
1.73 Molar
Step-by-step explanation:
The formula is Molarity=moles of solute/liters of solution, which can be written in whatever way you prefer, and examples include: M=N/V or M=mol/L.
M=N/V
M=
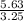
Divide 5.63 by 3.25. When you calculate this, you get 1.73, therefore your answer is 1.73 molar.