Answer:
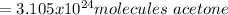
Step-by-step explanation:
Hello!
In this case, given the volume of acetone, it is possible to compute the mass and next the moles via its molar mass (58.08 g/mol) as shown below:
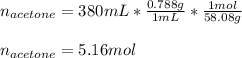
Then, via the Avogadro's number it is possible to compute the acetone molecules as follows:
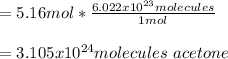
Best regards!