Answer:
Ph of the enzyme -catalyzed reaction = 6.77
Step-by-step explanation:
Given data:
concentration phosphate buffer = 0.1 m
pka. = 6.86
concentration of acid in the reaction = 0.005 m
Determine the Ph at the end of the reaction
For a balanced buffer solution ; Acid amount = base amount
first we will determine the concentration of acid and base in relation to the buffer
acid = 0.1 / 2 = 0.05
base = 0.1 /2 = 0.05
The chemical reaction of the buffer can be expressed as attached below
PH = pka + log
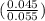 =
=
= 6.86 + log ( 0.8181 ) = 6.77