The change in internal energy when 2 moles of CO are converted to 2 moles of CO₂ at 1 atm and 25 °C is -566.0 kJ
How to calculate the change in the internal energy?
We'll begin by calculating the work done. This is shown below:
Equation:
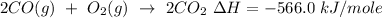
- Mole of CO converted = 2 moles
- Mole of CO₂ produced = 2 moles
- Pressure (P) = 1 atm
- Work done (W) = ?
From the above, we can see that the moles converted is the same. Thus, the change in volume, ΔV is zero. Therefore, we have
W = -PΔV
= -1 × 0
= 0 J
Now, we shall calculate the change in internal energy. Details below
- Heat released (Q) = -566.0 KJ
- Work done (W) = 0 J = 0 KJ
- Change in Internal energy (ΔU) =?
ΔU = Q + W
= -566.0 + 0
= -566.0 KJ