Answer: 1.96×10²⁴
Step-by-step explanation:
Since we are given the grams (mass) of Na and are being asked to find the atoms (particles) of Na, we can use stoichiometry with 2 ratios to identify the number of atoms in sodium.
We are going from grams → moles → atoms in this question
First, set up your first ratio. Since we are going from grams to moles in this first ratio, the first ratio would be 1 mole divided by the molar mass of the substance.
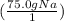
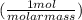
The molar mass of sodium (Na) is 23 g/mol or amu
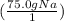
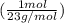
= 3.26 mol Na (you don't have to round this early, but for the sake of simplicity, I am rounding it to the appropriate number of significant figures)
Now we can convert moles to atoms. This ratio will be Avogadro's number divided by 1 mole.
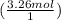
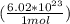
= 1.96252×10²⁴ atoms of Na
Since the information we were given (75.0 g of Na) has 3 significant figures, round 1.96252 to 3 sig figs.
= 1.96×10²⁴ atoms of Na