Answer:
Step-by-step explanation:
I know Avogadro's number to be 6.022 * 10²³ molecules/mol, but I will give the answer for both mine and your given value.
We are converting from moles to molecules, and we will do that by using Avogadro's number and our given number of moles:
3.87 mol (
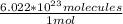 ) = 2.33 * 10²⁴ molecules
) = 2.33 * 10²⁴ molecules
3.87 mol (
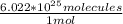 ) = 2.33 * 10²⁶ molecules
) = 2.33 * 10²⁶ molecules
I hope this helps! :)