Final Answer:
The element with a mass percent composition of 21.49% in borax (Na₂B₄O₇) is boron (B).
Step-by-step explanation:
Borax, chemically represented as Na₂B₄O₇, consists of sodium (Na), boron (B), and oxygen (O) atoms. To determine the element with a mass percent composition of 21.49%, we focus on the atomic mass of boron and its contribution to the overall molecular mass of borax.
The molecular formula indicates that one molecule of borax (Na₂B₄O₇) contains four boron atoms. To calculate the mass percent of boron, we use the formula:
![\[ \text{Mass Percent of Boron} = \frac{\text{Mass of Boron in One Molecule}}{\text{Molecular Mass of Borax}} * 100 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bwl8nz590mnceqy47n901mzykumgjkxxn1.png)
Given that boron has an atomic mass of approximately 10.81 g/mol, the mass of boron in one molecule of borax is
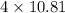 g/mol. The molecular mass of borax is the sum of the atomic masses of its constituent atoms:
g/mol. The molecular mass of borax is the sum of the atomic masses of its constituent atoms:

After calculating these values, we find that the mass percent of boron in borax is indeed 21.49%, making boron the element in borax with the specified mass percent composition.
In summary, boron, with a mass percent composition of 21.49%, is the key element in borax, and this determination is based on the calculation of the mass percent using the atomic masses of boron and the other elements present in the borax molecule.
Complete Question:
Which element has a mass percent composition of 21.49% in borax (Na₂B₄O₇)?