The final temperature of the mixture is approximately
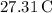 .
.
To find the final temperature of the mixture, we'll use the principle of conservation of energy. The heat lost by the warmer water will be equal to the heat gained by the cooler water. We'll assume no heat is lost to the surroundings.
The heat
 gained or lost by a substance is given by the formula:
gained or lost by a substance is given by the formula:
![\[ Q = mc\Delta T \]](https://img.qammunity.org/2024/formulas/physics/high-school/viivvm5fwe6r8lu77r18zmjvcc3t5mezpa.png)
where:
-
 is the mass of the substance,
is the mass of the substance,
-
 is the specific heat capacity of the substance (for water,
is the specific heat capacity of the substance (for water,
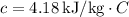 ),
),
-
 is the change in temperature.
is the change in temperature.
Let's denote:
-
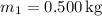 (mass of the cooler water),
(mass of the cooler water),
-
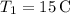 (initial temperature of the cooler water),
(initial temperature of the cooler water),
-
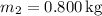 (mass of the warmer water),
(mass of the warmer water),
-
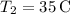 (initial temperature of the warmer water),
(initial temperature of the warmer water),
-
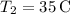 (final temperature of the mixture).
(final temperature of the mixture).
The heat lost by the warmer water is:
![\[ Q_{\text{lost}} = m_2 c (T_2 - T_f) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/xqho6m9nzncwjbwjhb252a50scs0a5376n.png)
The heat gained by the cooler water is:
![\[ Q_{\text{gained}} = m_1 c (T_f - T_1) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/rr4bhov0kxej0b0xlyogizby30jrs8e39n.png)
Since the heat lost by the warmer water is equal to the heat gained by the cooler water, we have:
![\[ m_2 c (T_2 - T_f) = m_1 c (T_f - T_1) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/veuhlavuupbmst5ptn99u9myy6e0agygqh.png)
Solving for
 , the equation becomes:
, the equation becomes:
![\[ m_2 (T_2 - T_f) = m_1 (T_f - T_1) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/hlpjszg2otkt2epygusg0zsx2hpkxhcy0h.png)
![\[ m_2 T_2 - m_2 T_f = m_1 T_f - m_1 T_1 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/vgp6m5lejfwtactbma3rx6llwg2zkiyxhi.png)
![\[ m_2 T_2 + m_1 T_1 = T_f (m_1 + m_2) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/5djmc9qbxvv3pmavuwpkbnqhrn9cea3q0j.png)
![\[ T_f = (m_2 T_2 + m_1 T_1)/(m_1 + m_2) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/uxha4unooptdlnyjbvbn76mgz4jv9l4dyg.png)
Substituting the values:
![\[ T_f = (0.800 * 35 + 0.500 * 15)/(0.800 + 0.500) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/76802kafy7gknhvuf6xvklt1k4nwnc4vhu.png)
Now, let's calculate the final temperature
 .
.
Let's calculate the final temperature
 manually:
manually:
![\[ T_f = (0.800 * 35 + 0.500 * 15)/(0.800 + 0.500) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/76802kafy7gknhvuf6xvklt1k4nwnc4vhu.png)
![\[ T_f = (28 + 7.5)/(1.3) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/wlzmry6606vyjay0zumvzy2bb8oilhy32a.png)
![\[ T_f = (35.5)/(1.3) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/r10vn92f0gcrbho4hivk55yzpp0sjak171.png)
![\[ T_f \approx 27.31 \, \text{C} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/49zopaq9kgixqgfsm94ghrn00y3xqfm0c8.png)
So, the final temperature of the mixture is approximately
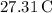 .
.