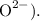To determine the oxide compound that an element is most likely to form based on its photoelectron spectrum, we need to consider the electron configuration as revealed by the spectrum.
A photoelectron spectrum displays the energy levels of electrons in an atom, and this information helps in understanding the valence electrons of the element.
Here's the general approach to deduce the likely oxide compound:
1.Identify Valence Electrons: The photoelectron spectrum shows peaks corresponding to electrons in different energy levels. The electrons in the outermost peaks are the valence electrons.
2. Determine Oxidation State: The number of valence electrons helps in predicting the common oxidation states. Elements tend to form compounds in a way to achieve a stable electron configuration, like that of a noble gas.
3. Predict the Oxide Compound: Based on the oxidation state, we can predict the formula of the oxide. For example, an element with two valence electrons (like calcium) would likely form an oxide with the formula
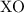 , as it would typically lose two electrons to oxygen (which gains two electrons to form
, as it would typically lose two electrons to oxygen (which gains two electrons to form