Answer:
The
 -carboxyl group is closer to the
-carboxyl group is closer to the
 -amino group than the side chain is.
-amino group than the side chain is.
Step-by-step explanation:
The
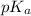 value gives an indication of the acidity of a group. A lower
value gives an indication of the acidity of a group. A lower
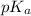 value indicates a stronger acid, meaning it donates its proton more easily.
value indicates a stronger acid, meaning it donates its proton more easily.
In glutamate, the
 -carboxyl group has a
-carboxyl group has a
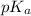 of 2.1, making it more acidic than the side chain carboxyl group with a
of 2.1, making it more acidic than the side chain carboxyl group with a
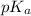 of 4.1.
of 4.1.
The difference in
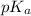 values between the
values between the
 -carboxyl group and the side chain carboxyl group can be attributed to the proximity of the
-carboxyl group and the side chain carboxyl group can be attributed to the proximity of the
 -carboxyl group to the
-carboxyl group to the
 -amino group. The positive charge on the
-amino group. The positive charge on the
 -amino group can stabilize the negative charge on the
-amino group can stabilize the negative charge on the
 -carboxyl group when it ionizes, making it more acidic.
-carboxyl group when it ionizes, making it more acidic.
Therefore, the correct statement is:
The
 -carboxyl group is closer to the
-carboxyl group is closer to the
 -amino group than the side chain is.
-amino group than the side chain is.