Answer:
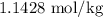
Step-by-step explanation:
To calculate the molality of a solution, we use the formula:
![\[ \text{Molality (m)} = \frac{\text{moles of solute}}{\text{mass of solvent (in kg)}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/xqpgyqjowp1l3qxj4o5a63xtlqk28uumw2.png)
Given:
Mass of
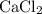 (solute) = 317 g
(solute) = 317 g
Mass of water (solvent) = 2.50 kg
First, we need to find the moles of
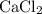 .
.
Molar mass of
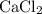 :
:
= Atomic weight of Ca + 2 × Atomic weight of Cl
= 40.08 g/mol (for Ca) + 2(35.45 g/mol) (for Cl)
= 40.08 g/mol + 70.90 g/mol
= 110.98 g/mol
Now, calculate the moles of
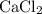 :
:
![\[ \text{moles of } \text{CaCl}_2 = \frac{\text{given mass}}{\text{molar mass}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/pi2eqdd846udtvfqcnbnk1dhp95locnhh6.png)
![\[ \text{moles of } \text{CaCl}_2 = \frac{317 \text{ g}}{110.98 \text{ g/mol}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/wiv4p9lwr3f6sbfezfplpzsh6xbsexj2v0.png)
![\[ \text{moles of } \text{CaCl}_2 = 2.857 \text{ moles} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/d6cw8q4989im1bj0amkmccu1fbi6cpi4ji.png)
Now, use the molality formula:
![\[ m = \frac{2.857 \text{ moles}}{2.50 \text{ kg}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/d0yekyc0kp989mod4aupan13nu5u72a7gk.png)
![\[ m = 1.1428 \text{ mol/kg} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bb6a2vue151kuxu85zqp4lff1bwl6yjgzj.png)
Therefore, the molality of the solution is
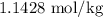 or approximately
or approximately
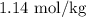 .
.