Answer:
unsolvable
Step-by-step explanation:
Molarity is defined by the equation:
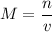
where
 is the number of moles of solute and
is the number of moles of solute and
 is the total volume of the solution (in liters).
is the total volume of the solution (in liters).
We are given that 10.8% of the weight of an H₂O₂ solution is H₂O₂ (and the rest is water, H₂O).
To figure out how many moles of H₂O₂ there would be in a 1kg solution, we can first find the molar mass of H₂O₂.
H₂O₂ ⇒ 2(1.008) + 2(15.999) = 34.014 g/mole
We know that there should be 108g of H₂O₂ in a 1kg solution, and we can convert this mass to moles using the molar mass we just solved for:
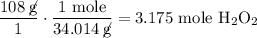
However, since the density of the solution is not given, we cannot accurately calculate the molarity.