Answer: 3.00% error
Step-by-step explanation:
% error =
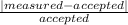 x 100%
x 100%
Our measured value is 58.2 g/mol and our accepted value is 60.0 g/mol. The absolute value is used in the numerator since we cannot have a negative value for %.
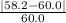 x 100% = 0.03 x 100%
x 100% = 0.03 x 100%
= 3.00% error
Bonus tip: there are many different terms for measured and accepted, below are a couple that I see most. The measured value is the value we got in a lab while the accepted value is the value that we would get if we were in completely ideal conditions for the lab.
- Different terms for measured: actual, experimental
- Different terms for accepted: known, theoretical
I hope this helps! :)