Answer: 226.6 mL FeBr₂ solution
Step-by-step explanation:
This is a unit conversion problem, and we are given a mass of FeBr₂ in grams, the formula weight of FeBr₂, and the molarity of the FeBr₂ solution. Molarity is in mol/L, so we will convert from g FeBr₂ → mol FeBr₂ → L FeBr₂ solution → mL FeBr₂ solution. I typed it 2 different ways in case the fractions are difficult to read. :)
65.0g FeBr₂ (
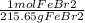 )(
)(
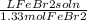 )(
)(
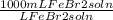 ) = 226.6 mL FeBr₂ soln
) = 226.6 mL FeBr₂ soln
65.0g FeBr₂(1 mol FeBr₂/215.65g FeBr₂)(L FeBr₂ soln/1.33 mol FeBr₂)(1000 mL/L) = 226.6 mL FeBr₂
If it's asking about significant figures for your final answer, I would go with 227 mL FeBr₂ solution, since two of our given values had 3 significant figures (65.0 and 1.33), and we use the same number of significant figures as the least precise given value, or the value with the least number of decimal places.
I hope this helped! :)