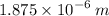Answer:
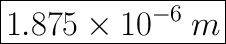
Step-by-step explanation:
The wavelength of the emitted photon can be found by using the Rydberg's equation which is given by;

where
 is the wavelength in m
is the wavelength in m
 is the final energy level
is the final energy level
 was the initial energy level
was the initial energy level
R is the Rydberg's constant which is 1.097 × 10⁷ m-¹
From the question
n1 = 4
n2 = 3
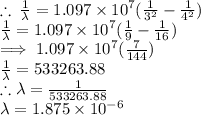
We have the final answer as