Answer: You can plug in the values into these equations to get the speed of the electrons.
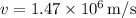
Step-by-step explanation:
First, we need to calculate the energy of one photon using the formula:

Where:
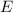 is the energy of the photon.
is the energy of the photon.
 is Planck's constant. (
is Planck's constant. (
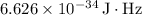 )
)
 is the frequency of light.
is the frequency of light.
Given:

Plugging in the values:
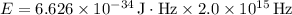
Next, we'll calculate the total energy of the batch of photons:
Given:

Using the energy of one photon, we can determine the number of photons in the batch:

The kinetic energy (KE) of the emitted electrons can be found using:
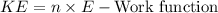
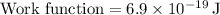
Finally, using the kinetic energy, we can determine the speed of the electrons using:
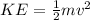
Where:
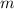 is the mass of an electron.
is the mass of an electron.

 is the speed of an electron.
is the speed of an electron.
Rearranging for
 :
:
