To calculate the mass of the aluminum pellets, we'll use the density formula:
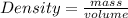
Given that the density of aluminum is
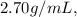 and the volume of the combined aluminum pellets and water is
and the volume of the combined aluminum pellets and water is
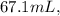 we can calculate the volume of water as well by subtracting the initial volume of the water (50.0 mL) from the combined volume:
we can calculate the volume of water as well by subtracting the initial volume of the water (50.0 mL) from the combined volume:
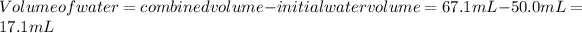
Now, let's calculate the mass of the aluminum pellets using the density formula:
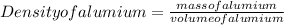
Solving for the mass of aluminum:
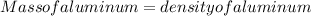 ×
×
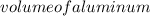
We can substitute the given values:
Mass of aluminum = density of aluminum × volume of aluminum
We can substitute the given values:
mass of aluminum = (2.70 g/mL) × (67.1 mL - 17.1 mL)
mass of aluminum = 2.70 g/mL × 50.0 mL
mass of aluminum = 135 g
So, the mass of the aluminum pellets is