Answer:
Approximately
 (rounded to two significant figures, assuming that
(rounded to two significant figures, assuming that
 .)
.)
Step-by-step explanation:
At the bottom of a column of liquid, the pressure from the liquid would be:
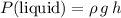 ,
,
Where:
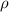 is the density of this liquid,
is the density of this liquid,
 is the gravitational field strength, and
is the gravitational field strength, and
 is the (vertical) height of the liquid column.
is the (vertical) height of the liquid column.
In a liquid barometer, the liquid pressure at the bottom of the column should be equal to air pressure outside the barometer.
The height of the liquid in this barometer can be found in the following steps:
- Find the value of the atmospheric pressure.
- Set the liquid pressure at the bottom of the barometer to be equal to the atmospheric pressure. Solve this equation for the height of the liquid column.
- Ensure that all quantities are measured in standard units. Substitute the values into the expression for the height of the liquid column and evaluate.
The atmospheric pressure at sea level on Earth is approximately
 (
(
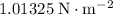 .)Thus,
.)Thus,
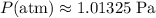 .
.
Equate the pressure at the bottom of the liquid column with the atmospheric pressure to obtain:
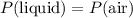 .
.
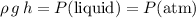 .
.
Rearrange this equation to find an expression for the height of the liquid column:
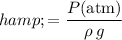 .
.
The standard unit for pressure is
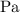 , which is equivalent to
, which is equivalent to
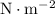 . The standard unit for density is
. The standard unit for density is
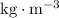 . The standard unit for gravitational field strength is
. The standard unit for gravitational field strength is
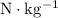 (equivalent to
(equivalent to
 .)
.)
Ensure that all quantities are measured in standard units. Evaluate to find the value for the height of the liquid column:
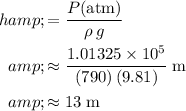 .
.
(Rounded to two significant figures.)
In other words, the height of this liquid column would be approximately
 .
.