The mass of the unit cell is approximately
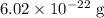 .( three significant figures).
.( three significant figures).
To calculate the mass of the unit cell, we first need to determine the number of moles of tantalum atoms in one unit cell. The mass of one mole of tantalum atoms (molar mass) is given as 180.95 g/mol.
The unit cell of a crystal structure often contains more than one atom. In the case of a body-centered cubic (BCC) unit cell, there are two atoms per unit cell.
The calculation is as follows:
1. Determine the number of moles of tantalum atoms in one unit cell:
![\[ \text{Moles of Ta atoms} = \frac{\text{Number of atoms per unit cell}}{\text{Avogadro's number}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ofeeve3xodmzpdj8z1llei61c2g0o2zqht.png)
For BCC, there are 2 atoms per unit cell.
![\[ \text{Moles of Ta atoms} = \frac{2}{6.022 * 10^(23) \, \text{mol}^(-1)} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/gquawpwhu9ohzad9bblivzcniqcqlm364i.png)
2. Calculate the mass of the unit cell:
![\[ \text{Mass of unit cell} = \text{Moles of Ta atoms} * \text{Molar mass of tantalum} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/mzmlp0ttdfe81ed87vv3z7xn3hreb9nuib.png)
![\[ \text{Mass of unit cell} = \left(\frac{2}{6.022 * 10^(23) \, \text{mol}^(-1)}\right) * 180.95 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/momu1fy0x4gqmdhoiaasgdqacvy78ac4rd.png)
Let's calculate the mass of the unit cell using the given expression:
![\[ \text{Mass of unit cell} = \left(\frac{2}{6.022 * 10^(23) \, \text{mol}^(-1)}\right) * 180.95 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/momu1fy0x4gqmdhoiaasgdqacvy78ac4rd.png)
![\[ \text{Mass of unit cell} = (2 * 180.95)/(6.022 * 10^(23)) \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/s0oso59f7ggf9i0061332ulcwkempjf7cw.png)
![\[ \text{Mass of unit cell} \approx (361.90)/(6.022 * 10^(23)) \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/gi1dtyu6q10vipxuyyoo6co70df5uxyag0.png)
Now, calculate this expression:
![\[ \text{Mass of unit cell} \approx 6.018 * 10^(-22) \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jsrvkmvaq4ie50busycpmhemzl1o4762ad.png)
Rounding to three significant figures, the mass of the unit cell is approximately
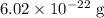 .
.