1) The ground state energy is 400 zJ
2) The first excited state is 750 zJ
3) A photon will be absorbed for the transition
4) The wavelength is 3 *
 m
m
What is energy transition in atoms?
When an electron absorbs energy, it can move to a higher energy level. This typically occurs when the atom absorbs a photon with energy equal to the energy difference between the initial and final energy levels of the electron. This process is associated with absorption spectra.
We have that;
ΔE = 1050 zJ - 400 zJ
= 650 zJ
Then;
ΔE = hc/λ
650 *
 = 6.6 *
= 6.6 *
 * 3 *
* 3 *
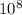 /λ
/λ
λ = 6.6 *
 * 3 *
* 3 *
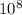 /650 *
/650 *

= 3 *
 m
m