Answer: 5 x
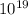 (atoms)
(atoms)
Step-by-step explanation:
According to Avogadro's law, at STP,
number of particles(atoms) =
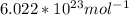 * n
* n
in which n is number of mol
Using Ideal Gas Law:
PV = nRT,
n = PV/RT
(Plug in values wrt STP)
=

(Put it in the calculator,)
= 4.159 x
 mol
mol

Getting back to our Avogadro's law, substitute:
number of particles(atoms) =
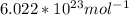 * (4.159 x
* (4.159 x
 mol
mol
 )
)
= 2.5 x
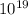
 ,
,
since there are 2 atoms in each
 particle, multiply our answer by 2 and we get:
particle, multiply our answer by 2 and we get:
5 x
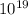 (particles)
(particles)