Answer:
Approximately
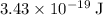 assuming that the wavelength was measured in vacuum.
assuming that the wavelength was measured in vacuum.
Step-by-step explanation:
Given the wavelength, the energy of this photon can be found in the following steps:
- Find the frequency
 of this photon from wave speed
of this photon from wave speed
 and wavelength
and wavelength
 using the equation
using the equation
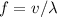 .
. - Find the energy of the photon from its frequency using the equation
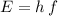 , where
, where
 is Planck's Constant.
is Planck's Constant.
Apply unit conversation and ensure that wavelength is measured in standard units:
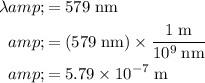 .
.
The speed of electromagnetic waves is the same as the speed of light. Assume that the wavelength of this photon was measured in vacuum, where the speed of light in vacuum is
 . Given that wavelength is
. Given that wavelength is
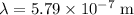 , the frequency of this photon would be:
, the frequency of this photon would be:
 .
.
Look up the value of Planck's Constant:
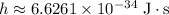 . Apply the equation
. Apply the equation
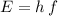 to find the energy of this photon from its frequency:
to find the energy of this photon from its frequency:
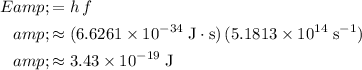 .
.
In other words, the energy of this photon would be approximately
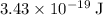 .
.