Answer:
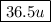
Step-by-step explanation:
Hydrochloric acid is

To find the molecular weight of a compound, we need simply the add the atomic weights of the elements multiplied by their atomicity.
Here, we have 1 atom of H and 1 atom of Cl in one molecule of HCl. Atomic weight of H is 1 and Cl is approx 35.5. Then molecular weight of HCl is:
= 1 + 35.5
= 36.5
Molecular weight is a relative quantity, so it has no units. But conventionally we write amu or u in the end to represent it as molecular weight. So molecular weight of HCl is 36.5 u