Answer:
The molar mass of a substance is the mass (in grams per mole) of 6.022 x
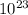 atoms, molecules, or formula units of that substance.
atoms, molecules, or formula units of that substance.
Step-by-step explanation:
In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass, the molecular mass, or the formula mass, respectively.