The entropy change for the system can be calculated using the formula:
ΔS = ΣnSf - ΣnSi
Where:
ΔS is the entropy change
ΣnSf is the sum of the entropy of the final state of each component
ΣnSi is the sum of the entropy of the initial state of each component
In this case, we are producing 1.00 mol of artificial air consisting of 79% N2 and 21% O2. To determine the entropy change, we need to calculate the entropy of the final state and the entropy of the initial state.
Step 1: Calculate the entropy of the final state
Since we are producing 1.00 mol of artificial air, we have:
nN2f = 0.79 mol (79% of 1.00 mol)
nO2f = 0.21 mol (21% of 1.00 mol)
Using the given value of S298 = 223.066 J/mol-K for Cl2(g), we can calculate the entropy of the final state:
ΣnSf = nN2f * S298(N2) + nO2f * S298(O2)
Step 2: Calculate the entropy of the initial state
We don't have the initial state information directly given in the question. However, we can assume that the initial state consists of separate N2 and O2 gases before they are combined to form the artificial air.
Using the given formula for C (J/mol-K):
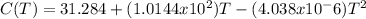
We can calculate the entropy of N2 and O2 at 298 K and 1500 K:
Si = ∫C(T)dT
For N2:
nN2i = 0.79 mol (79% of 1.00 mol)
![Si(N2) = ∫[31.284 + (1.0144 x 10^2)T - (4.038 x 10^-6)T^2]dT (from 298 K to 1500 K)](https://img.qammunity.org/2024/formulas/chemistry/high-school/71b54flh6gxj3vzkqo7utw9mv065jfz8ec.png)
For O2:
nO2i = 0.21 mol (21% of 1.00 mol)
Si(O2) = ∫[31.284 + (1.0144 x 10^2)T - (4.038 x 10^-6)T^2]dT (from 298 K to 1500 K)
Step 3: Calculate the entropy change
Now we can calculate the entropy change using the formula:
ΔS = ΣnSf - ΣnSi
Make sure to substitute the values calculated in steps 1 and 2 into the equation to obtain the final value of ΔS.
Keep in mind that the pressure is kept constant at 1.00 atm throughout the process.